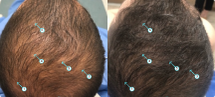
This study was prepared for DS Laboratories on May 21, 2017. We will continue to publish our clinical studies on our products to show results and efficacy of our products. You can see before and after pictures
Spectral.DNC-N Study - Males With Thinning Hair & Alopecia (Product Efficacy)
Translation missing: en.blogs.article.author_on_date_html
1. Summary
1.1 Conclusions
The results of this study showed that Spectral.DNC-N solution was effective in the promotion of hair growth and in the cessation of hair loss in males suffering with androgenetic alopecia and thinning hair. Subjects were also evaluated for adverse events.
1.2 Study Objectives
The primary goal of the study was to assess the safety and effectiveness of Spectral.DNC-N solution in the promotion of hair growth and in the cessation of hair loss in males.
1.3 Study Design
The study was designed as a double-blind trial conducted at the Miami Rescue Mission Clinic in Miami, FL. This study was not placebo-controlled. However, the application of the DNC-N solution was done by the lab technicians in order to observe the efficacy under proper use. Therefore study participants had to visit the clinic every day for 90 days including weekends and holidays. The solution was kept in a white container simply marked “Solution A”. Neither the lab technician nor the study participants knew what was being applied to their scalps. Global photographs were taken at baseline and then again at 90 days.
1.4 Subject Population and Demographics
The study population included males between the ages of 25 and 55 years with who had been experiencing active hair loss. Subjects who were primarily number 3 and 4 on the Norwood Scale were favored for the study due to the possibility of faster results. A few volunteers with more advanced baldness were included also. A total of 25 subjects participated in the study but only 18 completed the study. Five volunteers were removed from the study due to low adherence to the treatment schedule.
1.5 Methods
Prior to beginning the study subjects had their scalp evaluated for dermatological conditions that could potential disqualify them. All volunteers had healthy scalps and were enrolled in the study. A global photograph was taken from the crown area and a second photograph was taken of the top of the head to track frontal areas. Subjects then had to appear at the office every day for the technician to apply the solution to the scalp under controlled conditions. The improvements in the target site were observed using high resolution photography.
1.6 Results
Below are examples of effectiveness that seen at the completion of the study. The photographs show the image at baseline and then a secondary image collected at 90 days. One adverse even was observed as irritation on one of the subjects.
Table 1 summarizes the subjects own assessment of overall hair regrowth mean change from baseline in %. 16 out of 18 subjects had an increase in mean terminal hair growth. A few had a significant increase. Two subjects did not respond to the treatment.
Subjects who responded to the treatment had early stage hair loss or thinning hair. Two of the subjects had a dramatic improvement.
Subjects Assessment of Overall Hair Regrowth Mean Change From Baseline in %
No Growth 11%
Minimal Growth 17%
Moderate Growth 55%
Dense Growth 17%
1.7 Adverse events
One subject developed irritation approximately 4 weeks into the study. The irritation appeared as small red bumps in the treatment area. The technician was told to reduce the dosage in half and within 2 days the irritation cleared. No other adverse events were observed.
2. Description of Subjects
2.1 Inclusion Criteria
• Subject must have had presence of miniaturized hair in the target area.
• Subject must have been experiencing active hair loss, within the last 12 months.
• In good general health.
• A Norwood Scale classification of 2 through 5.
• Between 25 and 55 years of age at the time of enrollment.
• Able to read, understand, and provide signed informed consent after the nature of the
study has been fully explained and before any procedures dictated by this protocol were
performed.
• Subjects must have been willing and able to adhere to the daily office visits, first visit
being between 8am and noon and the second visit between 5pm and 8pm. These visits
are required 7 days a week including weekends and holidays. Subjects were informed
that if they miss more than one visit per week that they would be dropped from the
study.
2.2 Exclusion Criteria
• An active malignancy of any type or history of any malignancy including any malignancy
in the treatment area in the past five years.
• Any active skin infection in the scalp area or scarring in the target area.
3.1 Visit 1 – Screening (Day -14)
Potential subjects read and signed informed consent. Following the consent process, demographic and background information was collected, including age, and ethnic origin. Additionally, all medications, including over-the-counter drugs, taken within 6 months prior to the start of the study were recorded. An evaluation was also conducted and recorded to determine that subjects did not exhibit signs of skin/scalp conditions that would preclude participation in the study. Global digital images were taken. The global image was used to assist the investigator in completing the global assessment.
The subject sat in a chair (the height of the chair remained consistent throughout the study) and digital images were collected. The digital images were standardized for lighting, camera angle, and position of subject’s head in each digital image to achieve similar camera angle and relative image size. Throughout the study the investigator monitored each of the subjects for signs of scalp irritation or any other adverse events.
4. Commentary
The results observed indicate further that hair loss has multiple pathologies and that an effective treatment may not be effective for all subjects. This study was particularly interesting because 2 of the subjects had remarkable growth that exceeded all expectations as far as what is typically considered possible for advanced stages of baldness. Simultaneously, 2 other subjects did not respond at all. Therefore the mechanism of action of Spectral.DNC-N may be of particularly great value to some subjects but may not address the underlying etiology in others. This is certainly an indication that a tailored approach is needed in some cases and that some of the more challenging cases can be treated successfully with a more tailored approach.
SUBJECT #2
SUBJECT #3
SUBJECT #4
SUBJECT #5
SUBJECT #6